last highlighted date: 2024-04-05
Highlights

- Note: scope of iec62304 vs iec82304 it means software only medical devices and especiallly for other health use are under scope of iec8204. wellness


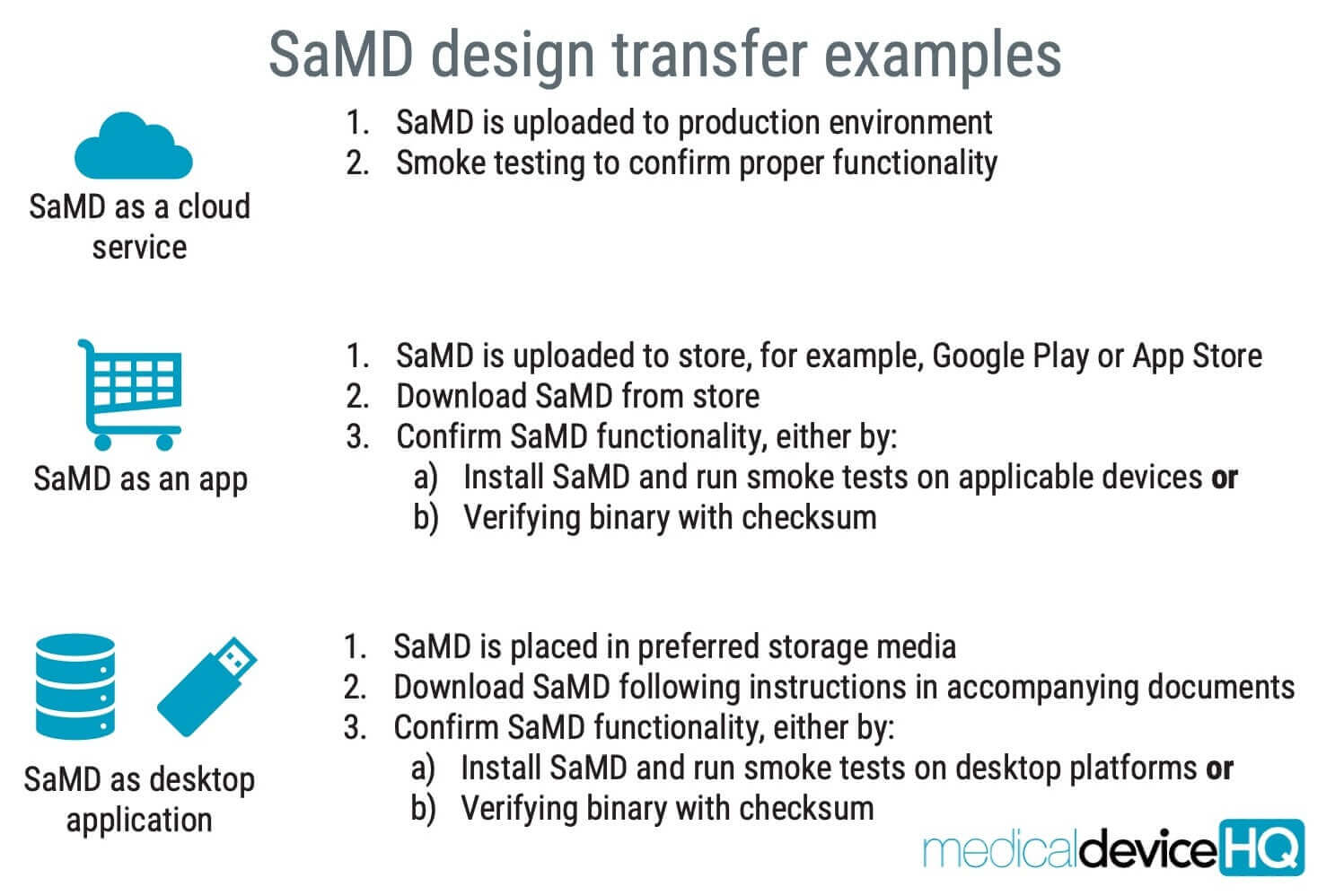
- Note: smoke test means quick test if it still works

- The standard IEC 82304-1 applies to health software products designed to operate on general computing platforms and are intended to be placed on the market without dedicated hardware.
- software intended to be used specifically for managing, maintaining, or improving health of individual persons, or the delivery of care
- IEC 82304-1 is a product standard that complements the process standard IEC 62304 when working with health software. The difference between product and process standards is illustrated below.
- While continuous integration can be considered a good practice when working with SaMD, a word of caution is justified when considering continuous deployment.
- The end-user is not a guinea pig
- Note: :)
- Whether design transfer applies to software and SaMD, in particular, can be debatable. Neither IEC 82304-1 nor IEC 62304 mentions anything about design transfer, but it is an important activity of ISO 13485 (clause 7.3.8):
- In 2019, the FDA received over 200 submissions for AI/ML-based SaMD.
- Regulators acknowledge the need to update AI-based applications and eventually re-train based on new data – without requiring new submissions. However, such updates are still expected to happen under controlled circumstances and are not supposed to be autonomously initiated by the device.
- Note: they know we need it, but not now! 2024
- AI and ML algorithms are often used in SaMD to analyse large amounts of data quickly and accurately. For instance, they can be used to detect patterns or anomalies in medical images, predict patient outcomes based on historical data, or even recommend treatment options.

- By 2025, the global market for SaMD is projected to reach $86.5 billion.